

Uneedle is a design and manufacturing company of innovative products for superficial injection that is transforming the healthcare sector through silicon microneedles, intradermal injections, and suprachoroidal injections. Stablished in 2008, the UT spin-off is located in Enschede and remains a close collaborator with the University of Twente to continuously improve their products and their manufacturing capabilities.
The company delivers high-quality and reliable products that are easy to learn for medical professionals requiring injection into superficial tissues. Their designs are focused on creating needles that are very short, sharp, precise, and possible to manufacture following international medical product regulations.
Their intradermal focus allows the medication to be applied directly into the dermis, the layer underneath the skin, where it comes in direct contact with the immune systems right away. This form of medicine application requires five to ten times less product compared to muscular injection. Thus allowing for a more efficient use of costly resources such as cancer treatments or vaccines. Moreover, an intradermal approach is less likely to cause pain in the area of injection after the application, proving to be a better experience for every patient.
Groundbreaking innovations (range of products)
The pharmaceutical and healthcare sector has seen great advancements in the products and technologies used for treating patients. The COVID pandemic highlighted the challenges of supplying sufficient medical goods to meet market demand, safe injection procedures, and efficient use of vaccines and medications. Uneedle has faced these challenges by developing innovative products and proprietary production technologies that result in unique designs that can benefit patients all around the world.
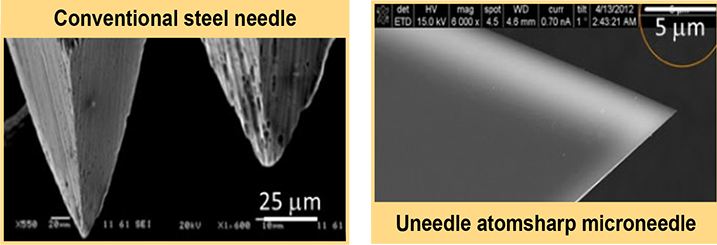
Silicon microneedle
Uneedle has redesigned the conventional steel needle that doctors and nurses use for treatment and vaccinations. Steel needles, sharpened through grinding and polishing techniques, face a limit on the sharpness and bevel length that can be achieved during manufacturing. Uneedle’s technology overcomes this challenge through the use of silicon and its unique properties for microscale geometries. Extra sharp silicon needles leverage the crystal planes of the material to create an optimised geometry for the needle. Through semiconductor etching processes, and without grinding or polishing, the cutting edges of the needle tip are always “atom sharp” regardless of the bevel angle and length. The Uneedle technology enables sharp needles with an ultra-short bevel that, due to the silicon’s properties, do not suffer from wear, are smooth for lubricant-free use, and are free of grinding-induced particles. Uneedle’s silicon needle has open the way to develop new groundbreaking products designed to improve the medical injection industry with products such as Bella-mu and Bella-vue.
Intradermal injection
Bella-mu, the perpendicular injection product from Uneedle simplifies intradermal administration of up to 1 mL and offers an improved alternative to ordinary hypodermic needles. Intradermal application can effectively target the immune system, provide direct access to skin, and allow a fast systemic uptake of medications. For these reasons, it is essential to provide medical professionals with a product that they can easily learn how to use and serves as a reliable method of intradermal injection. Improving these needles would benefit doctors and patients during vaccination, allergy therapies, skin and aesthetic treatments, diabetes medication, cancer vaccines, and other medications that aim for enhanced immune response. The ultra-short silicon needle technology from Uneedle enables a reliable perpendicular injection that medical professionals can easily perform while the patient experiences less needle fear and pain from the injection.
Suprachoroidal injection
Bella-vue, is the suprachoroidal injection product for the ocular route of drug administration. Safe and effective access to this area can benefit glaucoma and retinal treatments, prosthesis implementations, and medicine injection. Designing advanced high-quality products for suprachoroidal injection can revolutionise the field of ophthalmology. Manufacturing high-quality needles will allow medical professionals to use this minimally invasive approach for delivering personalised treatments to the back of the eye. The multiple advantages of this injection route have led Uneedle to develop Bella-vue, along with the pharmaceutical industry, to improve the treatment experience of major retinal diseases.
Manufacturing with the Highest Quality
Uneedle’s in-house department of product development has earned them patents for their unique production process. Manufacturing needles is a challenging task that requires the latest technologies to meet the high-quality standards of CE certifications, ISO 13485 certified, and FDA-approval. Uneedle patented work allows them to achieve the high standards from healthcare certifications, meet the needs from their clients, and support doctors and patients that will be benefiting from such products during treatment. These certifications guarantee the quality management of the medical products they offer thus making their product appropriate for the market and clinical investigations.
Their manufacturing facilities, involving innovative manufacturing processes, makes them Good Manufacturing Practice (GMP) compliant which translates to delivering products of high quality, appropriate for their intended purpose, and meet authorisation requirements from the European Medicines Agency (EMA).
One of the challenges in manufacturing of medical devices is to ensure the highest quality and safety of the product. Uneedle achieves this, for example, by using Semicon’s industry standard assembly line, which includes a fully automated die attachment robot.
Product traceability represents another challenge to overcome in the medical industry as LOT traceability is essential for customers and authorities. Uneedle is developing methods and software to address this, such as in the participation within NXTGEN HighTech programme where research is being conducted to offer a “full traceability” environment. This means that each product will be given a unique number which can be used to provide full manufacturing traceability.
The future for healthcare and manufacturing
Healthcare and pharmaceutical sectors are constantly changing through new technologies, new treatments, new materials, and new medical research. Continuing to innovate is essential to improve the daily work of doctors and nurses, as well as every person facing health issues. Uneedle understands the importance of this as they work to deliver goods of the utmost quality through an efficient production structure. Looking forward, Uneedle aims to increase their production capability to deliver up to five million needles a year to their international customers. Such production goals will bring benefits for their company, research partners, and millions of patients across the globe.

