The Twilight of Lead Acid Batteries
Navigating the Transition from Legacy to Innovation
Introduction: The End of an Era
For more than 150 years, lead acid batteries (LABs) have been a cornerstone of global industry, powering everything from automobiles to critical infrastructure. Their reliable performance and relatively low cost made them the go-to choice for a variety of applications. However, the world of energy storage is evolving rapidly, and lead acid batteries are being overshadowed by newer, more efficient technologies. Yet, around 70% of the global energy storage market is still leveraging on LABs [1]. Exploring the history, technology, and ongoing relevance of LABs offers valuable insights into their role in the evolving energy market, while also highlighting the potential for industries to adapt and thrive in the shifting landscape of energy storage.
The Fundamentals of Lead Acid Batteries
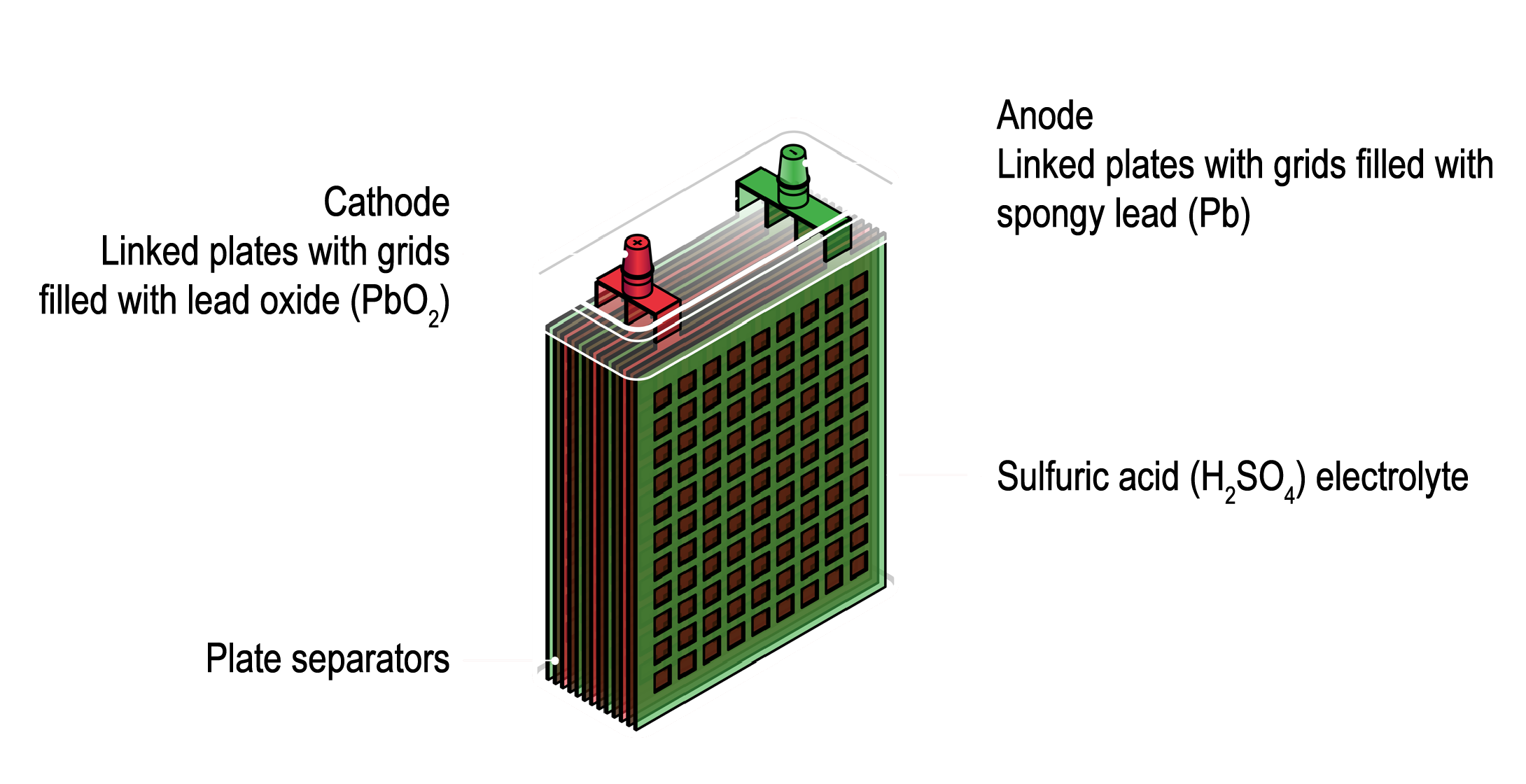
First developed in 1859, LABs are one of the oldest types of rechargeable batteries. They operate through a chemical reaction between lead dioxide and sponge lead in a sulfuric acid electrolyte, which generates electrical energy. This chemical formula is principally rechargeable and reversible, as represented below:
PbO2+Pb+2H2SO4→2PbSO4+2H2O
Despite their relatively low energy density compared to newer commercial alternatives, and the need for regular maintenance, LABs are known for their ability to deliver high surge currents, making them indispensable in various applications over the decades. Most notably, lead acid batteries were the dominant energy storage technology in the automotive industry for many decades, until the advent of lithium-ion batteries (LIBs) transformed the market.

The Evolution of Lead Acid Batteries: Past to Present
Lead acid batteries have been pivotal across various industries, with applications evolving alongside technological advancements. Historically, their high current delivery made them perfect for starting, lighting, and ignition systems in automobiles, as backup power systems for telecommunications and uninterruptible power supply (UPS) applications, as well as signalling and lighting systems in railways and other transport infrastructure.
Today, LABs remain widely used as backup power solutions in residential, commercial, and industrial settings where cost-effectiveness is essential. They continue to play a significant role in off-grid systems, particularly for storing renewable energy in cost-sensitive regions, and are commonly used in off-grid applications such as powering construction equipment and material handling vehicles like forklifts in warehouses. LABs are also integral to the energy grid, providing reliability for UPS systems and maintaining a presence in certain automotive applications.
The Decline: Why Lead Acid Batteries Are Phasing Out
Once the backbone of energy storage, LABs are being challenged by emerging technologies that deliver significantly higher energy density, require less maintenance, and offer longer lifecycles between charges. For example, while LABs have a theoretical energy density limit of just 30% to 40%, LIBs boast around 90%, a key factor in their dominance across 90% of new energy storage installations [2].

Environmentally, the key technological components of lead acid batteries (LABs)—lead and sulfuric acid—are toxic materials that pose significant risks if not properly managed. Despite these concerns, LABs still account for about 30% of the energy storage market, largely due to their cost-effectiveness and reliability [3]. However, this number is rapidly declining as the energy storage market changes, highlighting a shift away from LABs.
This raises an important question: what happens when fully rechargeable and reliable LABs end up in waste streams? Improper disposal can result in severe environmental consequences, including lead contamination, acid leakage, and the release of other harmful heavy metals. Recycling presents an attractive alternative, as up to 96% of their materials, including lead, can be recovered and reused [4]. However, when LABs that are not severely damaged or degraded are discarded rather than reconditioned, it represents a missed opportunity to repurpose them as low-cost energy storage solutions.
The Future of Lead Acid Batteries: Reconditioning, Rechargeability, and Stability
Despite the decline in new applications, LABs still hold significant potential through reconditioning, which leverages their rechargeability and capitalizes on their inherent stability. The renewable energy market is facing challenges due to the mismatch between energy production peaks and demand peaks, which highlights the need for more affordable energy storage solutions. The reconditioning process is relatively straightforward and offers direct benefits by delaying disposal and providing a cost-effective option for non-critical systems and low-cost energy storage. LABs are reliable and stable, performing effectively across a wide temperature range, and their low discharge rates extend their shelf life, making them an ideal candidate for repurposing rather than disposal.

A Glimmer of Hope: the ReLAB project
In a rapidly evolving energy market, the ReLAB project shines a green light on the untapped potential of decommissioned lead-acid batteries (LABs). By reconditioning these batteries for alternative energy storage, the project not only extends their lifespan but also offers a cost-effective solution for local and off-grid energy needs. As the demand for sustainable and affordable energy storage grows, ReLAB demonstrates that even as LABs are being phased out, they can still play a crucial role in meeting energy challenges through innovative reuse, providing a practical and impactful way to bridge the gap between energy production and demand.
Conclusion: The Lasting Legacy
While the energy storage landscape is shifting towards newer technologies, LABs continue to hold value through their reconditioning potential, reliable rechargeability, and inherent stability. By capitalizing on these strengths, businesses can extend the life of existing assets, reduce costs, and enhance energy independence. Although the peak of LAB use is behind us, a significant number of LABs remain in service or are nearing decommissioning. By reconditioning these batteries, we unlock substantial energy storage potential, ensuring they continue to play a vital role in the energy ecosystem. Let’s leverage on the past to reliably power the future.
